 |
HOW FirePro® WORKS IN A FIRE | |||||
| FirePro® aerosol extinguishes fire by inhibiting the chain chemical reactions present in combustion on a molecular level. | ||||||
| Theory of Fire – Four elements must be present for a fire to occur, a fire is extinguished by removing one or more of these four elements from the combustion process. | ||||||
| The Chemistry of Fire | ||||||
    |
||||||
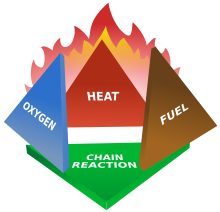 Fire is a redox (oxidation-reduction) chemical process of combustion involving rapid oxidation of a fuel source at an elevated temperature, accompanied by the release of energy and the production of heat and light and gaseous by-products. As an energy release mechanism, it is the exothermic reaction involving oxidation that produces heat. Oxidation and reduction always occur in tandem in a redox process. If one substance gains oxygen (oxidation), then a second substance must also be present to lose oxygen (reduction). The first substance is the reducing agent (oxidised) and the second substance is the oxidising agent (oxidiser). Reducing agents remove oxygen from another substance, and oxidising agents give oxygen to another substance. The combustion process begins when a fuel source is heated beyond its ignition temperature in the presence of an oxidant, with this molecular energy creating a self-sustaining chemical chain reaction of radicals when the energy it produces is greater than or equal to the energy needed for continued burning.Fire is a redox (oxidation-reduction) chemical process of combustion involving rapid oxidation of a fuel source at an elevated temperature, accompanied by the release of energy and the production of heat and light and gaseous by-products. As an energy release mechanism, it is the exothermic reaction involving oxidation that produces heat. Oxidation and reduction always occur in tandem in a redox process. If one substance gains oxygen (oxidation), then a second substance must also be present to lose oxygen (reduction). The first substance is the reducing agent (oxidised) and the second substance is the oxidising agent (oxidiser). Reducing agents remove oxygen from another substance, and oxidising agents give oxygen to another substance. The combustion process begins when a fuel source is heated beyond its ignition temperature in the presence of an oxidant, with this molecular energy creating a self-sustaining chemical chain reaction of radicals when the energy it produces is greater than or equal to the energy needed for continued burning. Fire is a redox (oxidation-reduction) chemical process of combustion involving rapid oxidation of a fuel source at an elevated temperature, accompanied by the release of energy and the production of heat and light and gaseous by-products. As an energy release mechanism, it is the exothermic reaction involving oxidation that produces heat. Oxidation and reduction always occur in tandem in a redox process. If one substance gains oxygen (oxidation), then a second substance must also be present to lose oxygen (reduction). The first substance is the reducing agent (oxidised) and the second substance is the oxidising agent (oxidiser). Reducing agents remove oxygen from another substance, and oxidising agents give oxygen to another substance. The combustion process begins when a fuel source is heated beyond its ignition temperature in the presence of an oxidant, with this molecular energy creating a self-sustaining chemical chain reaction of radicals when the energy it produces is greater than or equal to the energy needed for continued burning.Fire is a redox (oxidation-reduction) chemical process of combustion involving rapid oxidation of a fuel source at an elevated temperature, accompanied by the release of energy and the production of heat and light and gaseous by-products. As an energy release mechanism, it is the exothermic reaction involving oxidation that produces heat. Oxidation and reduction always occur in tandem in a redox process. If one substance gains oxygen (oxidation), then a second substance must also be present to lose oxygen (reduction). The first substance is the reducing agent (oxidised) and the second substance is the oxidising agent (oxidiser). Reducing agents remove oxygen from another substance, and oxidising agents give oxygen to another substance. The combustion process begins when a fuel source is heated beyond its ignition temperature in the presence of an oxidant, with this molecular energy creating a self-sustaining chemical chain reaction of radicals when the energy it produces is greater than or equal to the energy needed for continued burning.
The Four Elements. Oxidising Agent Oxygen is the most common oxidising agent. Oxygen supports combustion but does not burn. Normal air contains 21% oxygen; and the higher the concentration of oxygen in the air, the more intensely a fire will burn. However, a fire can burn without the presence of oxygen if another oxidising agent is present, e.g. nitrates, peroxides, chlorine, etc. Heat is produced by an exothermic reaction (a chemical reaction that produces more energy than needed for the reaction to occur, causing the excess energy to be released as heat). Heat transfers from an area of higher temperature to an area of lower temperature by three principal means: conduction, convection and radiation. Conduction is the transfer of heat between substances that are in direct contact with each other. Convection only occurs in liquids and gases. Liquid and gas expand and become less dense as they are heated. This causes them to rise, being displaced by colder and denser liquid or gas. This is in turn heated and also rises. The risen liquid or gas cools and falls, with the resultant convection current occurring until a uniform temperature is attained. Radiation is the transfer of heat energy through electromagnetic radiation in the infra-red part of the spectrum, between substances that are not in direct contact with each other. Extinguishing a Fire. a fire can be extinguished by removing any one of the four elements. In practical fire-fighting terms, this translates as starvation, cooling, smothering, and interference: Starvation is the process of depriving the fire of fuel, i.e. combustible materials.
Cooling is depriving the fire of heat, e.g. by applying a substance such as water that will absorb heat from the fire and reduce the temperature below the critical level needed to sustain the fire. Smothering is the process of depriving the fire of the oxygen needed to sustain the combustion process. Interference is the process of applying extinguishing agents to the fire that inhibit the chemical chain reaction at the molecular level. All fire suppression operate on these principles, with their various extinguishing agents removing one or more of the four elements from the fire. For example, water fire extinguishers work by cooling and quenching a fire (removing the element of heat from it) and carbon dioxide fire extinguishers work by displacing oxygen at the source of the fire and replacing it with an inert gas (removing the element of oxygen from it) and they also have a limited cooling effect. In addition to extinguishing a fire by cooling it or depriving it of oxygen, a fire can be extinguished by removing its fuel source or by applying extinguishing agents that inhibit the combustion process at the molecular level.
|
||||||